15+ Calculate Zeff For A Valence Electron In An Oxygen Atom
Web Calculate zeff for a valence electron in an oxygen atom. Oxygen has 8 protons but has 6 valence electrons so it.

What Is Effective Nuclear Charge And The Periphery Of Nitrogen Atom When An Extra Electron Is Added In The Formation Of An Anion
Web Which of the following compounds contains oxygen has no absorption above 3100 cm1 and none between 1650 and 1780 cm1.

. Calculate zeff for a valence electron in an oxygen atom. Zeff 345 Submit Previous. Besides the formula for calculating the effective nuclear charge of a single electron is as follows.
Two atoms share an electron. Calculate zeff for a valence electron in an oxygen. The nucleus of a.
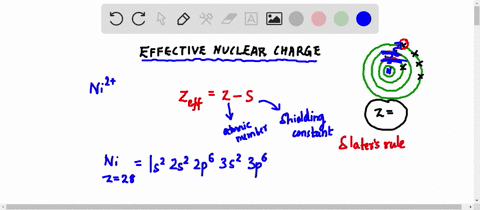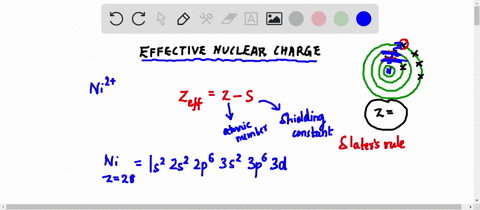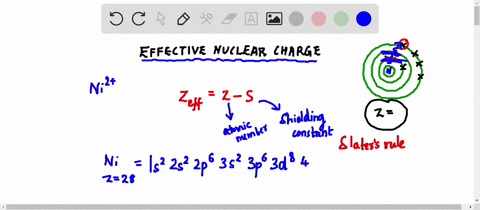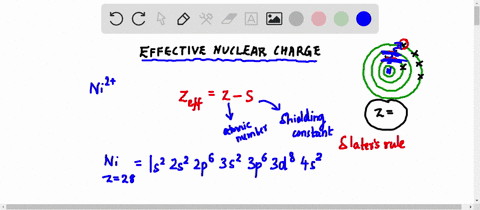
Web Up to 256 cash back Get the detailed answer. Web Zeff Z S 8 345 455. To determine the value for S start by writing out the electron configuration for the atom.
Web Up to 256 cash back Get the detailed answer. View Available Hint s IVO ΟΙ ΑΣΦ. Use Slaters Rules O 1s2 2s 2p6 So shielding S 035 x 5 085 x 2.
Web Use the periodic table to determine the number of protons in a copper atom. Web The effective nuclear charge on a valence electron of carbon is 325 while the effective nuclear charge on a valence electron in oxygen is 345. Express your answer numerically.
The effective nuclear charge experienced by a valence electron in an O atom is 455. The magnesium will create an ionic bond with the oxygen by giving its 2 electrons to form. Web Calculate Zeff for a valence electron in an oxygen atom.
2 Answers Use Slaters Rules O. Calculate Zeff for one valence electron in an oxygen atom. Part A Calculate Zeff for a valence electron in an oxygen atom.
Web Calculate Zeff for a valence electron in an oxygen atom. Calculate the for the valence electron in an oxygen atom. Who are you and how did you know that.
Web An atom is always more stable when it has 8 valence electrons. Jpdvolleybal1326 jpdvolleybal1326 11172017 Chemistry. Web Two atoms attain equal electronegativities.
Web Calculate Zeff for a valence electron in an oxygen atom. Web Effective Nuclear Charge is equal to the atomic number protons subtracted by the shielding electrons or valence electrons. Express your answer numerically.
When an electron drops into a lower-energy orbital energy is released in the form of. Calculate the for the valence electron in an oxygen. Web An atom is always more stable when it has 8 valence electrons.
QABy tamdoanJuly 14 20210 Comment thanks in advance. Web The electron from a hydrogen atom drops from an excited state into the ground state. One atom becomes more electronegative than another atom.

Solved Part A Calculate Zeff For A Valence Electron In An Oxygen Atom Express Your Answer Numerically View Available Hint S Azd Zeff 3 45 Submit Previous Answers Incorrect Try Again 4 Attempts Remaining

Solved A Write The Electron Configuration For L I And Estimate The Effective Nuclear Charge Experienced By The Valence Electron B The Energy Of An Electron In A One Electron Atom Or Ion Equals

Solved Question 2 Arrange The Following Atoms In Order Of Increasing Effective Nuclear Charge That Their Valence Electrons Experience Al K N Sc Note The Atom For Which Zeff Has The Smallest Value

Solved Part A Calculate Zeff For A Valence Electron In An Oxygen Atom Express Your Answer Numerically View Available Hint S Azd Zeff 3 45 Submit Previous Answers Incorrect Try Again 4 Attempts Remaining

Welcome To Chem Zipper Com Effective Nuclear Charge Z Or Zeff Slater S Rule Screening Effect Or Shielding Effect

Ions Effective Nuclear Charge Of Oxygen Atom O Vs Oxygen Anion O2 Chemistry Stack Exchange
Solved Calculate The Effective Nuclear Charge On A Valence Electron In An Oxygen Atom

Calculate The Effective Nuclear Charge For The Outermost Electron Of Oxygen Atom

Solved Calculate Zeff For Oxygen Atom On Outermost Shell Electron Remember Contribution For Valance Lectrons Are Ns And Np 0 35 N 1 Contribulion Is 0 85 The Second Ionization Energy Of Some Period Elements Are
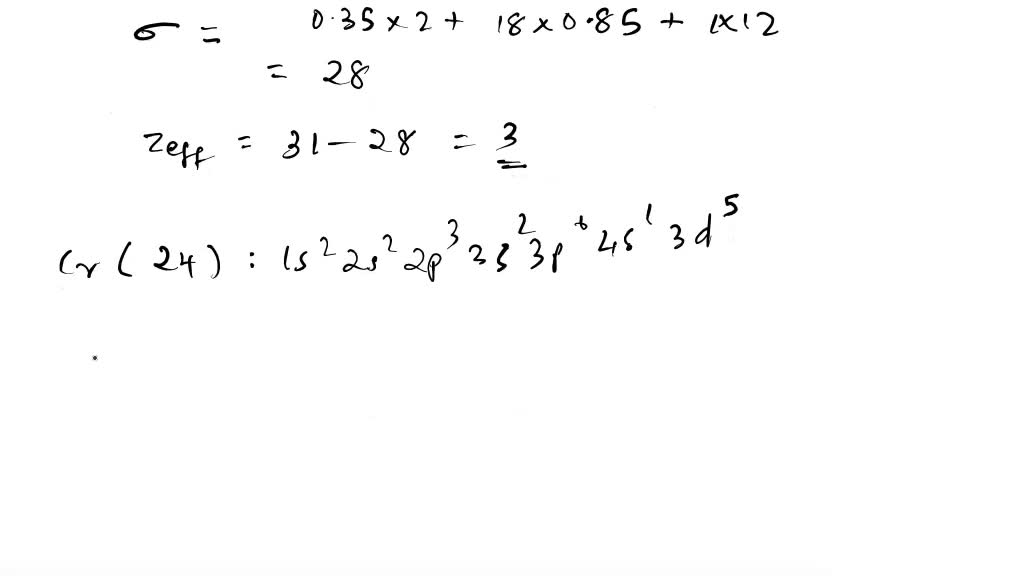
Solved Calculate Zeff For Oxygen Atom On Outermost Shell Electron Remember Contribution For Valance Lectrons Are Ns And Np 0 35 N 1 Contribulion Is 0 85 The Second Ionization Energy Of Some Period Elements Are
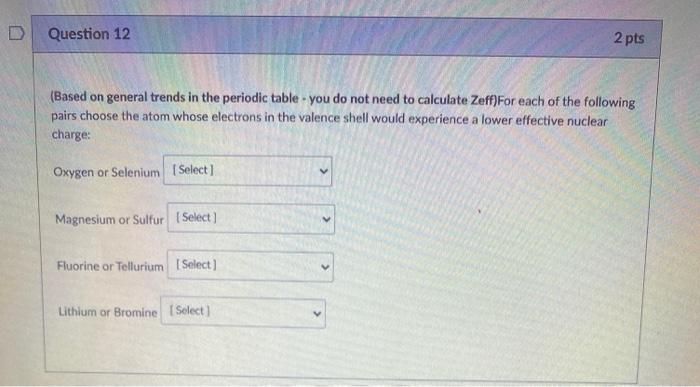
Solved Question 12 2 Pts Based On General Trends In The Chegg Com

Solved Which Would Experience A Higher Effective Nuclear Chegg Com
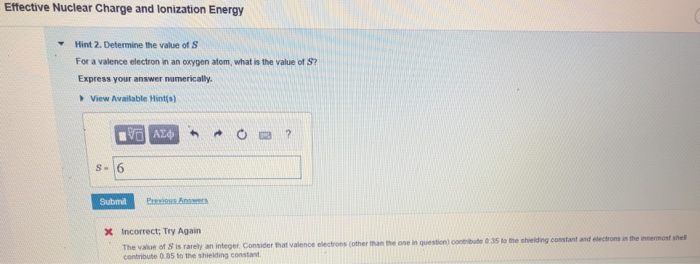
Solved Effective Nuclear Charge And Lonization Energy Hint Chegg Com

Calculate The Effective Nuclear Charge Of The Last Electron In An Atom The Electronic Configuration Is 1s 2 2s 2 2p 6 3s 2 3p 5

How To Calculate The Effective Nuclear Charge Of An Electron Youtube

Effective Nuclear Charge Eightfold
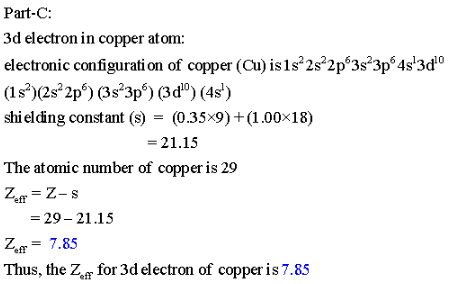
Part A Calculate Zeff For A Valence Electron In An Oxygen Atom Home Work Help Learn Cbse Forum